28.1 INTRODUCTION
The eye is a seat of a steady electric potential field that is quite unrelated to light stimulation. In fact, this field may be detected with the eye in total darkness and/or with the eyes closed. It can be described as a fixed dipole with positive pole at the cornea and negative pole at the retina. The magnitude of this corneoretinal potential is in the range 0.4-1.0 mV. It is not generated by excitable tissue but, rather, is attributed to the higher metabolic rate in the retina. The polarity of this potential difference in the eyes of invertebrates is opposite to that of vertebrates. This potential difference and the rotation of the eye are the basis for a signal measured at a pair of periorbital surface electrodes. The signal is known as the electro-oculogram, (EOG). It is useful in the study of eye movement. A particular application of the EOG is in the measurement of nystagmus, which denotes small movements of the eye. The resulting signal is called an electronystagmogram. It depends both on the visual system and the vestibular system and provides useful clinical information concerning each. Some details concerning the EOG as it relates to eye movement, including nystagmus, is contained in the following sections. The lens of the eye brings the illuminated external scene to a focus at the retina. The retina is the site of cells that are sensitive to the incident light energy; as with other peripheral nerve cells, they generate receptor potentials. The collective behavior of the entire retina is a bioelectric generator, which sets up a field in the surrounding volume conductor. This potential field is normally measured between an electrode on the cornea (contact-lens type) and a reference electrode on the forehead. The recorded signal is known as the electroretinogram (ERG). It may be examined both for basic science studies and for clinical diagnostic purposes. Though the electroretinogram is produced by the activity of excitable nervous tissue, and should therefore be discussed in Part IV, it is discussed in this chapter in connection with the electrooculogram to follow the anatomical division of bioelectromagnetism. It is, of course, more practical to discuss all electric signals originating in the eye after the anatomy and physiology of this organ are presented.
The lens of the eye brings the illuminated external scene to a focus at the retina. The retina is the site of cells that are sensitive to the incident light energy; as with other peripheral nerve cells, they generate receptor potentials. The collective behavior of the entire retina is a bioelectric generator, which sets up a field in the surrounding volume conductor. This potential field is normally measured between an electrode on the cornea (contact-lens type) and a reference electrode on the forehead. The recorded signal is known as the electroretinogram (ERG). It may be examined both for basic science studies and for clinical diagnostic purposes. Though the electroretinogram is produced by the activity of excitable nervous tissue, and should therefore be discussed in Part IV, it is discussed in this chapter in connection with the electrooculogram to follow the anatomical division of bioelectromagnetism. It is, of course, more practical to discuss all electric signals originating in the eye after the anatomy and physiology of this organ are presented.
28.2 ANATOMY AND PHYSIOLOGY OF THE EYE AND ITS NEURAL PATHWAYS
28.2.1 Major Components of the Eye
The eye and its major components are shown in Figure 28.1. Light enters the front of the eye at the cornea. Behind the cornea exists a transparent fluid called the aqueous humor. Its main function is to make up for the absence of vasculature in the cornea and lens by providing nutrients and oxygen. The aqueous also is responsible for generating a pressure of 20-25 mmHg, which inflates the eye against the relatively inelastic boundaries provided by the sclera and choroid. This ensures an appropriate geometrical configuration for the formation of clear images by the optical pathway. The lens is located behind the aqueous humor. Its shape and refractive index are controlled by the ciliary muscles. The lens completes the focusing of the light, begun at the cornea, on the retina. Between the lens and the retina is the vitreous chamber, which is filled with gel-like transparent material known as the vitreous humor. The center of the visual image is focused on the retina to the fovea, where visual accuracy is the highest. The retina contains photosensitive cells and several layers of neural cells. This combination generates action pulses relative to the visual image which passes out of the eye to the brain on the optic nerve (Rodieck, 1973).
The center of the visual image is focused on the retina to the fovea, where visual accuracy is the highest. The retina contains photosensitive cells and several layers of neural cells. This combination generates action pulses relative to the visual image which passes out of the eye to the brain on the optic nerve (Rodieck, 1973).

- Fig. 28.1 Horizontal section of the right human eye seen from above. The anteroposterior diameter averages 24 mm.
28.2.2 The Retina
A drawing of the major elements of the retinal cellular structure is shown in Figure 28.2. In this figure, light enters from the top and passes through the neural structure to the photoreceptors, which are the rods and cones. Just behind the rods and cones is the retinal pigment epithelium (RPE). Its major function is to supply the metabolic needs (as well as other supportive functions) of the photoreceptors. The rods respond to dim light, whereas the cones contribute to vision in bright light and in color. This area is the site of visual excitation (Charles, 1979). The initial step in the translation of light information from a spot of light into an electric signal propagating to the visual cortex takes place in the photoreceptors in a process known as transduction. This consists of the cis-trans isomerization of the carotenoid chromophore, which leads to a transient change in the membrane potential of the cell. The result consists of a graded response, seen as a hyperpolarization of the photoreceptor, and an electrotonic current linking the outer and inner segments. A photoreceptor is capable of transducing the energy of a single photon (about 4×10-12erg) into a pulsed reduction of axial current of about 1 pA lasting about 1 s with an energy equivalent of 2×10-7erg (Levick and Dvorak, 1986). Thus, a photoreceptor serves as a photomultiplier with an energy gain of some 105 times. The combined volume conductor signal from all photoreceptors contributes what is known as a late receptor potential (LRP).
The initial step in the translation of light information from a spot of light into an electric signal propagating to the visual cortex takes place in the photoreceptors in a process known as transduction. This consists of the cis-trans isomerization of the carotenoid chromophore, which leads to a transient change in the membrane potential of the cell. The result consists of a graded response, seen as a hyperpolarization of the photoreceptor, and an electrotonic current linking the outer and inner segments. A photoreceptor is capable of transducing the energy of a single photon (about 4×10-12erg) into a pulsed reduction of axial current of about 1 pA lasting about 1 s with an energy equivalent of 2×10-7erg (Levick and Dvorak, 1986). Thus, a photoreceptor serves as a photomultiplier with an energy gain of some 105 times. The combined volume conductor signal from all photoreceptors contributes what is known as a late receptor potential (LRP).
 The photoreceptors synapse with a horizontal cell and bipolar cell in what is known as the triad. The signal transmitted via the horizontal cell results in the inhibition of neighboring receptor cells (lateral inhibition) and, hence, an enhancement in contrast. The bipolar cell responds electrotonically with either a hyperpolarization or depolarization. The bipolar cells synapse with ganglion cells. This synaptic connection, however, is modulated by the amacrine cells. These cells provide negative feedback and thus allow regulation of the sensitivity of transmission from the bipolar to ganglion cells to suitable levels, depending on the immediate past light levels. At the ganglion cell the prior (slow) graded signals are converted into an action pulse that can now be conveyed by nerve conduction to the brain. The magnitude of the slow potential is used by the ganglion cell to establish the firing rate, a process sometimes described as converting from amplitude modulation to pulse-frequency modulation.
The photoreceptors synapse with a horizontal cell and bipolar cell in what is known as the triad. The signal transmitted via the horizontal cell results in the inhibition of neighboring receptor cells (lateral inhibition) and, hence, an enhancement in contrast. The bipolar cell responds electrotonically with either a hyperpolarization or depolarization. The bipolar cells synapse with ganglion cells. This synaptic connection, however, is modulated by the amacrine cells. These cells provide negative feedback and thus allow regulation of the sensitivity of transmission from the bipolar to ganglion cells to suitable levels, depending on the immediate past light levels. At the ganglion cell the prior (slow) graded signals are converted into an action pulse that can now be conveyed by nerve conduction to the brain. The magnitude of the slow potential is used by the ganglion cell to establish the firing rate, a process sometimes described as converting from amplitude modulation to pulse-frequency modulation.
 The region of the retinal pigment epithelium and the posterior portion of the photoreceptors (rods and cones) is called the outer nuclear layer. The region of contact of the photoreceptors with the bipolar cells is known as the outer plexiform layer (OPL). The main function of the OPL appears to be signal processing. Since there are 100×106 rods and 6×106 cones but only 1×106 ganglion cells, a marked convergence must take place in the course of signal processing. The bipolar and amacrine cells form the inner nuclear layer. The region of contact of the bipolar and amacrine cells with the ganglion cells is known as the inner plexiform layer (IPL). The amacrine cells play a role similar to the horizontal cells in the OPL, except that the amacrine cells act in the temporal domain whereas the horizontal cells affect the spatial domain.
The region of the retinal pigment epithelium and the posterior portion of the photoreceptors (rods and cones) is called the outer nuclear layer. The region of contact of the photoreceptors with the bipolar cells is known as the outer plexiform layer (OPL). The main function of the OPL appears to be signal processing. Since there are 100×106 rods and 6×106 cones but only 1×106 ganglion cells, a marked convergence must take place in the course of signal processing. The bipolar and amacrine cells form the inner nuclear layer. The region of contact of the bipolar and amacrine cells with the ganglion cells is known as the inner plexiform layer (IPL). The amacrine cells play a role similar to the horizontal cells in the OPL, except that the amacrine cells act in the temporal domain whereas the horizontal cells affect the spatial domain.
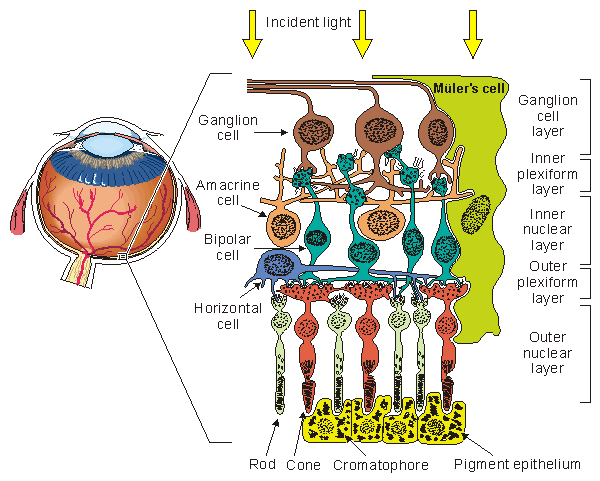
- Fig. 28.2 The retinal cellular structure.
28.3 ELECTRO-OCULOGRAM
28.3.1 Introduction
Emil du Bois-Reymond (1848) observed that the cornea of the eye is electrically positive relative to the back of the eye. Since this potential was not affected by the presence or absence of light, it was thought of as a resting potential. In fact, as we discuss in a subsequent section, it is not constant but slowly varying and is the basis for the electro-oculogram (EOG). This source behaves as if it were a single dipole oriented from the retina to the cornea. Such corneoretinal potentials are well established and are in the range of 0.4 - 1.0 mV. Eye movements thus produce a moving (rotating) dipole source and, accordingly, signals that are a measure of the movement may be obtained. The chief application of the EOG is in the measurement of eye movement.
This source behaves as if it were a single dipole oriented from the retina to the cornea. Such corneoretinal potentials are well established and are in the range of 0.4 - 1.0 mV. Eye movements thus produce a moving (rotating) dipole source and, accordingly, signals that are a measure of the movement may be obtained. The chief application of the EOG is in the measurement of eye movement.
 Figure 28.3 illustrates the measurement of horizontal eye movements by the placement of a pair of electrodes at the outside of the left and right eye (outer canthi). With the eye at rest the electrodes are effectively at the same potential and no voltage is recorded. The rotation of the eye to the right results in a difference of potential, with the electrode in the direction of movement (i.e., the right canthus) becoming positive relative to the second electrode. (Ideally the difference in potential should be proportional to the sine of the angle.) The opposite effect results from a rotation to the left, as illustrated. The calibration of the signal may be achieved by having the patient look consecutively at two different fixation points located a known angle apart and recording the concomitant EOGs. Typical achievable accuracy is ±2° , and maximum rotation is ±70° however, linearity becomes progressively worse for angles beyond 30° (Young, 1988). Typical signal magnitudes range from 5-20 µV/°.
Figure 28.3 illustrates the measurement of horizontal eye movements by the placement of a pair of electrodes at the outside of the left and right eye (outer canthi). With the eye at rest the electrodes are effectively at the same potential and no voltage is recorded. The rotation of the eye to the right results in a difference of potential, with the electrode in the direction of movement (i.e., the right canthus) becoming positive relative to the second electrode. (Ideally the difference in potential should be proportional to the sine of the angle.) The opposite effect results from a rotation to the left, as illustrated. The calibration of the signal may be achieved by having the patient look consecutively at two different fixation points located a known angle apart and recording the concomitant EOGs. Typical achievable accuracy is ±2° , and maximum rotation is ±70° however, linearity becomes progressively worse for angles beyond 30° (Young, 1988). Typical signal magnitudes range from 5-20 µV/°.
 Electro-oculography has both advantages and disadvantages over other methods for determining eye movement. The most important disadvantages relate to the fact that the corneoretinal potential is not fixed but has been found to vary diurnally, and to be affected by light, fatigue, and other qualities. Consequently, there is a need for frequent calibration and recalibration. Additional difficulties arise owing to muscle artifacts and the basic nonlinearity of the method (Carpenter, 1988). The advantages of this technique include recording with minimal interference with subject activities and minimal discomfort. Furthermore, it is a method where recordings may be made in total darkness and/or with the eyes closed. Today the recording of the EOG is a routinely applied diagnostic method in investigating the human oculomotor system. The application of digital computers has considerably increased the diagnostic power of this method (Rahko et al., 1980). In the following, we discuss in greater detail the two subdivisions of the electrooculography - the saccadic response and nystagmography.
Electro-oculography has both advantages and disadvantages over other methods for determining eye movement. The most important disadvantages relate to the fact that the corneoretinal potential is not fixed but has been found to vary diurnally, and to be affected by light, fatigue, and other qualities. Consequently, there is a need for frequent calibration and recalibration. Additional difficulties arise owing to muscle artifacts and the basic nonlinearity of the method (Carpenter, 1988). The advantages of this technique include recording with minimal interference with subject activities and minimal discomfort. Furthermore, it is a method where recordings may be made in total darkness and/or with the eyes closed. Today the recording of the EOG is a routinely applied diagnostic method in investigating the human oculomotor system. The application of digital computers has considerably increased the diagnostic power of this method (Rahko et al., 1980). In the following, we discuss in greater detail the two subdivisions of the electrooculography - the saccadic response and nystagmography.

Fig. 28.3 An illustration of the electro-oculogram (EOG) signal generated by horizontal movement of the eyes. The polarity of the signal is positive at the electrode to which the eye is moving.
28.3.2 Saccadic Response
Saccadic movements describe quick jumps of the eye from one fixation point to another. The speed may be 20 - 700°/s. Smooth movements are slow, broad rotations of the eye that enable it to maintain fixation on an object moving with respect to the head. The angular motion is in the range of 1 - 30°/s. The adjective pursuit is added if only the eye is moving, and compensatory if the eye motion is elicited by body and/or head movement. The aforementioned eye movements are normally conjugate that is, involve parallel motion of the right and left eye. In fact, this is assumed in the instrumentation shown in Figure 28.3; were this not the case , separate electrode pairs on the sides of each eye would become necessary. A normal saccadic response to a rapidly moving target is described in Figure 28.4. The stimulus movement is described here as a step, and eye movement speeds of 700°/s are not uncommon. The object of the oculomotor system in a saccade is to rapidly move the sight to a new visual object in a way that minimizes the transfer time.
A normal saccadic response to a rapidly moving target is described in Figure 28.4. The stimulus movement is described here as a step, and eye movement speeds of 700°/s are not uncommon. The object of the oculomotor system in a saccade is to rapidly move the sight to a new visual object in a way that minimizes the transfer time.
 The parameters commonly employed in the analysis of saccadic performance are the maximum angular velocity, amplitude, duration, and latency. The trajectory and velocity of saccades cannot voluntarily be altered. Typical values of these parameters are 400°/s for the maximum velocity, 20° for the amplitude, 80 ms for the duration, and 200 ms for the latency.
The parameters commonly employed in the analysis of saccadic performance are the maximum angular velocity, amplitude, duration, and latency. The trajectory and velocity of saccades cannot voluntarily be altered. Typical values of these parameters are 400°/s for the maximum velocity, 20° for the amplitude, 80 ms for the duration, and 200 ms for the latency.
 When following a target moving in stepwise jumps, the eyes normally accelerate rapidly, reaching the maximum velocity about midway to the target. When making large saccades (>25°), the eyes reach the maximum velocity earlier, and then have a prolonged deceleration. The movement of the eyes usually undershoots the target and requires another small saccade to reach it. Overshooting of the target is uncommon in normal subjects. Normally the duration and amplitude are approximately linearly correlated to each other. Several factors such as fatigue, diseases, drugs, and alcohol influence saccades as well as other eye movements.
When following a target moving in stepwise jumps, the eyes normally accelerate rapidly, reaching the maximum velocity about midway to the target. When making large saccades (>25°), the eyes reach the maximum velocity earlier, and then have a prolonged deceleration. The movement of the eyes usually undershoots the target and requires another small saccade to reach it. Overshooting of the target is uncommon in normal subjects. Normally the duration and amplitude are approximately linearly correlated to each other. Several factors such as fatigue, diseases, drugs, and alcohol influence saccades as well as other eye movements.
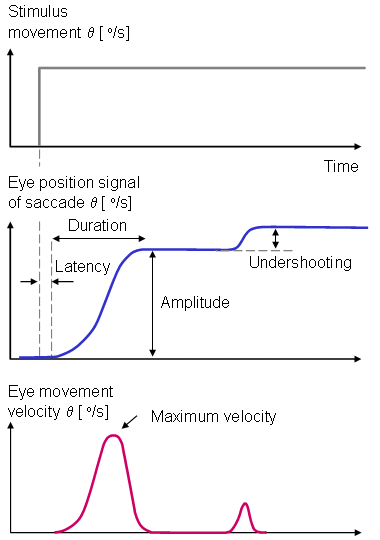
Fig. 28.4 An illustration of the eye movement response to a step stimulus (i.e., a spot of light whose horizontal position instantaneously shifts). After a latency the eye rapidly moves toward the new position, undershoots, and moves a second time. The movements are illustrative of saccades, and the parameters include latency, amplitude, velocity, duration, overshooting, and undershooting.
28.3.3 Nystagmography
Nystagmography refers to the behavior of the visual control system when both vestibular (balance) and visual stimuli exist. Nystagmoid movement is applied to a general class of unstable eye movements, and includes both smooth and saccadic contributions. Based on the origin of the nystagmoid movement, it is possible to separate it into vestibular and optokinetic nystagmus. Despite their different physiological origin, these signals do not differ largely from each other.Vestibular Nystagmus
Nystagmography is a useful tool in the clinical investigation of the vestibular system (Stockwell, 1988). The vestibular system senses head motion from the signals generated by receptors located in the labyrinths of the inner ear. Under normal conditions the oculomotor system uses vestibular input to move the eyes to compensate for head and body motion. This can occur with saccadic and/or pursuit motion (Figure 28.5A). If the vestibular system is damaged then the signals sent to the oculomotor system will be in error and the confusion experienced by the patient results in dizziness. Conversely, for a patient who complains of dizziness, an examination of the eye movements arising from vestibular stimuli can help identify whether, in fact, the dizziness is due to vestibular damage.
If the vestibular system is damaged then the signals sent to the oculomotor system will be in error and the confusion experienced by the patient results in dizziness. Conversely, for a patient who complains of dizziness, an examination of the eye movements arising from vestibular stimuli can help identify whether, in fact, the dizziness is due to vestibular damage.
 Inappropriate compensatory eye movements can easily be recognized by the trained clinician. Such an examination must be made in the absence of visual fixation (since the latter suppresses vestibular eye movements) and is usually carried out in darkness or with the patient's eye closed. Consequently, monitoring eye movement by EOG is the method of choice.
Inappropriate compensatory eye movements can easily be recognized by the trained clinician. Such an examination must be made in the absence of visual fixation (since the latter suppresses vestibular eye movements) and is usually carried out in darkness or with the patient's eye closed. Consequently, monitoring eye movement by EOG is the method of choice.
Optokinetic Nystagmus
Another example of nystagmoid movement is where the subject is stationary but the target is in rapid motion. The oculomotor system endeavors to keep the image of the target focused at the retinal fovea. When the target can no longer be tracked, a saccadic reflex returns the eye to a new target. The movements of the eye describe a sawtooth pattern, such as shown in Figure 28.5B. This is described as optokinetic nystagmus. This may also be provoked in the laboratory by rotating a cylinder with dark stripes on a light background in front of a person's eyes.
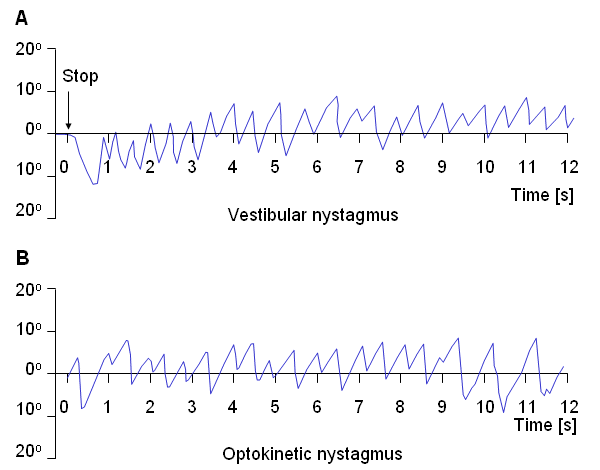
- Fig. 28.5 (A) An illustrative record of saccades arising from vestibular nystagmus.
 (B) An illustrative record of saccades arising from optokinetic nystagmus.
(B) An illustrative record of saccades arising from optokinetic nystagmus.28.4 ELECTRORETINOGRAM
28.4.1 Introduction
F. Holmgren (1865) showed that an additional time-varying potential was elicited by a brief flash of light, and that it had a repeatable waveform. This result was also obtained, independently, by Dewar and McKendrick (1873). This signal is the electroretinogram (ERG), a typical example of which is shown in Figure 28.6. It is clinically recorded with a specially constructed contact lens that carries a chlorided silver wire. The electrode, which may include a cup that is filled with saline, is placed on the cornea. The reference electrode is usually placed on the forehead, temple, or earlobe. The amplitude depends on the stimulating and physiological conditions, but ranges in the tenths of a millivolt. The sources of the ERG arise in various layers of the retina, discussed above. These sources are therefore distributed and lie in a volume conductor that includes the eye, orbit, and, to an extent, the entire head. The recording electrodes are at the surface of this region. For the ERG one can identify the progressively changing layer from which different portions of the waveform arise, initiated by a brief light flash stimulus to the photoreceptors.
The sources of the ERG arise in various layers of the retina, discussed above. These sources are therefore distributed and lie in a volume conductor that includes the eye, orbit, and, to an extent, the entire head. The recording electrodes are at the surface of this region. For the ERG one can identify the progressively changing layer from which different portions of the waveform arise, initiated by a brief light flash stimulus to the photoreceptors.
 The earliest signal is generated by the initial changes in the photopigment molecules of the photoreceptors due to the action of the light. This usually gives rise to a positive R1 deflection followed by a negative R2 deflection, together making up the early receptor potential (ERP). This is followed, after around 2 ms, by the late receptor potential (LRP) mentioned earlier, which (combined with the remainder of the ERP) forms the main constituent of the a-wave, a corneo-negative waveform (see Figure 28.6). Both rods and cones contribute to the a-wave; however, with appropriate stimuli these may be separated. For example, a dim blue flash to the dark-adapted eye results in a rod ERG, whereas a bright red flash to a light-adapted eye results in a cone ERG.
The earliest signal is generated by the initial changes in the photopigment molecules of the photoreceptors due to the action of the light. This usually gives rise to a positive R1 deflection followed by a negative R2 deflection, together making up the early receptor potential (ERP). This is followed, after around 2 ms, by the late receptor potential (LRP) mentioned earlier, which (combined with the remainder of the ERP) forms the main constituent of the a-wave, a corneo-negative waveform (see Figure 28.6). Both rods and cones contribute to the a-wave; however, with appropriate stimuli these may be separated. For example, a dim blue flash to the dark-adapted eye results in a rod ERG, whereas a bright red flash to a light-adapted eye results in a cone ERG.
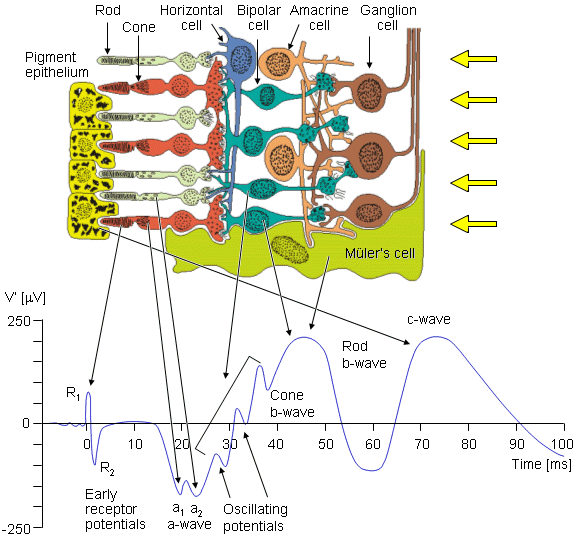
Fig. 28.6 The cells of the retina and their response to a spot light flash. The photoreceptors are the rods and cones in which a negative receptor potential is elicited. This drives the bipolar cell to become either depolarized or hyperpolarized. The amacrine cell has a negative feedback effect. The ganglion cell fires an action pulse so that the resulting spike train is proportional to the light stimulus level.
 The second maxima, which is corneo-positive, is the b-wave. To explain its origin we need to note that in the inner retinal layers there are Müler's cells. These cells are glial cells and have no synaptic connection to the retinal cells. The transmembrane potential of Müler's cells depends on its potassium Nernst potential, which is influenced by changes in the extracellular potassium. The latter is increased by the release of potassium when the photoreceptors are stimulated. In addition, the ganglion cell action pulse is associated with a potassium efflux. (The aforementioned electrophysiological events follow that described in Chapters 3 and 4.) The consequence of these events is to bring about a Müler's cell response. And it is the latter that is the source of the b-wave. Müler's cells can contribute to a b-wave from either cone or rod receptors separately.
The second maxima, which is corneo-positive, is the b-wave. To explain its origin we need to note that in the inner retinal layers there are Müler's cells. These cells are glial cells and have no synaptic connection to the retinal cells. The transmembrane potential of Müler's cells depends on its potassium Nernst potential, which is influenced by changes in the extracellular potassium. The latter is increased by the release of potassium when the photoreceptors are stimulated. In addition, the ganglion cell action pulse is associated with a potassium efflux. (The aforementioned electrophysiological events follow that described in Chapters 3 and 4.) The consequence of these events is to bring about a Müler's cell response. And it is the latter that is the source of the b-wave. Müler's cells can contribute to a b-wave from either cone or rod receptors separately.
 The c-wave is positive like the b-wave, but otherwise is considerably slower. It is generated by the retinal pigment epithelium (RPE) as a consequence of interaction with the rods.
The c-wave is positive like the b-wave, but otherwise is considerably slower. It is generated by the retinal pigment epithelium (RPE) as a consequence of interaction with the rods.
 The oscillatory potentials shown in Figure 28.6 are small amplitude waves that appear in the light-adapted b-wave. Although they are known to be generated in the inner retinal layer and require a bright stimulus, the significance of each wave is unknown. Some additional details are found in the paper by Charles (1979).
The oscillatory potentials shown in Figure 28.6 are small amplitude waves that appear in the light-adapted b-wave. Although they are known to be generated in the inner retinal layer and require a bright stimulus, the significance of each wave is unknown. Some additional details are found in the paper by Charles (1979).
 In retrospect, the sources that are responsible for the ERG and that lie within and behind the retina, are entirely electrotonic. They constitute a specific example of the receptor and generator potentials described and discussed in Chapter 5. This contrasts with the sources of the ECG in that the latter, which arise from cardiac muscle cells, are generated entirely from action pulses. Nevertheless, as described in Chapters 8 and 9, a double layer source is established in a cell membrane whenever there is spatial variation in transmembrane potential. Such spatial variation can result from a propagating action pulse and also from a spreading electrotonic potential. In both cases currents are generated in the surrounding volume conductor and the associated potential field may be sampled with surface electrodes that register the EOG and ERG. An examination of the ERG volume conductor is given below.
In retrospect, the sources that are responsible for the ERG and that lie within and behind the retina, are entirely electrotonic. They constitute a specific example of the receptor and generator potentials described and discussed in Chapter 5. This contrasts with the sources of the ECG in that the latter, which arise from cardiac muscle cells, are generated entirely from action pulses. Nevertheless, as described in Chapters 8 and 9, a double layer source is established in a cell membrane whenever there is spatial variation in transmembrane potential. Such spatial variation can result from a propagating action pulse and also from a spreading electrotonic potential. In both cases currents are generated in the surrounding volume conductor and the associated potential field may be sampled with surface electrodes that register the EOG and ERG. An examination of the ERG volume conductor is given below.
28.4.2 Volume Conductor Influence on the ERG
We have described the sources of the ERG lying in the retina (or the RPE) and being measured by a corneal and (say) temple electrode. To model this system requires a description of the volume conductor that links the source with its field. A first effort in this direction is the axially symmetric three-dimensional model of Doslak, Plonsey, and Thomas (1980) described in Figure 28.7. Because of the assumed axial symmetry, the model can be treated as two-dimensional - a large simplification in the calculation of numerical solutions. In this model, the following inhomogeneities were identified:The aqueous humor and vitreous body were assumed to constitute a single region of uniform conductivity since, in fact, they have nearly the same conductivity (σ1).
The sclera (σ2).
The extraocular region was considered to have a uniform conductivity, much the same as simplified models of the ECG consider the torso uniform (σ3).
The lens (σ4).
The cornea (σ5).
The air in front of the eye, which has a conductivity of zero (σ6).
The model includes the R-membrane, which lies at the same radius as the retina and continues to the cornea. This membrane was treated as a distribution of parallel RC elements (RR, RC).
The retina itself was assumed to be the location of a uniform double layer source, considered to extend over a hemisphere.
| Parameter | Structure | Value in model | Dimension |
| σ1 | Aqueous & Vitreous | 1.0 | 57 [S/cm] |
| σ2 | Sclera | 0.01 ... 0.15 | 57 [S/cm] |
| σ3 | Extraocular | 0.0005 ... 0.06 | 57 [S/cm] |
| σ4 | Lens | 0.08 ... 0.3 | 57 [S/cm] |
| σ5 | Cornea | 0.03 ... 0.86 | 57 [S/cm] |
| σ6 | Air | 0.0 | 57 [S/cm] |
| RR | R-membrane resistinv. | 1.67 ... 6.25 | 1/57 [Ω/cm²] |
| RC | 1/(2πCs) | 27.8 ... 58.8 | 1/57 [Ω/cm²] |
| RXC | Capacitive reactance | RC/frequency | |
|
Note: C is the R-membrane capacitance. Division of σi by 57 gives conductivity in [S/cm]. Multiplication of RR, RC, and RXC by 57 gives resistivity in [Ωcm²]. Source: Doslak, Plonsey, and Thomas (1980). | |||
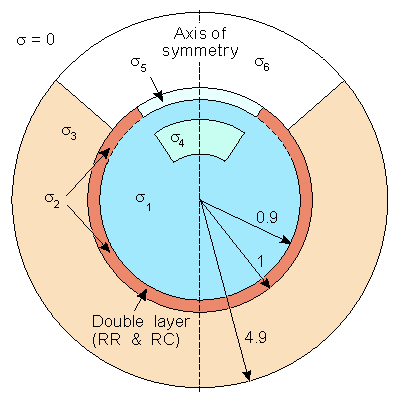
Fig. 28.7 The two-dimensional model depicting the ERG source and volume conductor inhomogeneities. The retina and R-membrane impedance are represented together by double layer and RR and RC, respectively. The other parameters correspond to the conductivities and are listed in Table 28.1.
In the model described by Figure 28.7 we seek the potential Φ that satisfies
  2Φ = 0 2Φ = 0 | (28.1) |
namely, Laplace's equation subject to the following boundary conditions: At all passive interfaces between regions of different conductivity the normal component of current density is continuous and the electric potential is continuous. For the retinal double layer, the normal component of current density is continuous, but the potential is discontinuous across this source by a value equal to the double layer strength (expressed in volts). Finally, for the R-membrane, the current density is also continuous, but there is a discontinuity in potential; this is given by the product of membrane impedance (Ωcm²) and the normal component of current density. Doslak, Plonsey, and Thomas (1980) solved this by locating a system of nodal points over the entire region and then using the method of finite differences and overrelaxation. Mathematical details are contained in Doslak, Plonsey, and Thomas (1982). The model was used by Doslak and Hsu (1984) to study the effect of blood in the vitreous humor on the ERG magnitude. They were able to establish that little effect on ERG magnitude could be expected from this condition.
28.4.3 Ragnar Granit's Contribution
Hermann von Helmholtz (1867) developed the theory of color vision on the basis of the ideas of English scientist Thomas Young (1802). He proposed that the human ability to discriminate a spectrum of colors is based on three different kinds of receptors which are sensitive to different wavelengths of light - red, green, and violet. The perception of other colors would arise from the combined stimulation of these elements. Ragnar Granit's first experiments in color vision, performed in 1937, employed the electroretinogram (ERG) to confirm the extent of spectral differentiation. Using the microelectrode, which he developed in 1939, he studied color vision further and established the spectral sensitivities of the three types of cone cells: blue, green, and red. These results he confirmed in a later study on color vision (Granit, 1955). Ragnar Granit shared the 1967 Nobel Prize with H. Keffer Hartline and George Wald "for their discoveries concerning the primary physiological and chemical visual processes in the eye."
Ragnar Granit's first experiments in color vision, performed in 1937, employed the electroretinogram (ERG) to confirm the extent of spectral differentiation. Using the microelectrode, which he developed in 1939, he studied color vision further and established the spectral sensitivities of the three types of cone cells: blue, green, and red. These results he confirmed in a later study on color vision (Granit, 1955). Ragnar Granit shared the 1967 Nobel Prize with H. Keffer Hartline and George Wald "for their discoveries concerning the primary physiological and chemical visual processes in the eye."
 A seminal study of the ERG was conducted by Ragnar Granit (1955). He recognized the distributed nature of the sources and designed experiments to block different parts in an effort to identify the major elements contributing to the waveform. He deduced the presence of three main components, namely PI, PII, and PIII. PI is a slowly developing positive potential and is associated with the c-wave. PII is also positive but develops more rapidly and is chiefly responsible for the b-wave. PIII is the negative component; its initial phase develops rapidly and is associated with the onset of the a-wave. The total ERG is found by superposition (summing) of PI+PII+PIII.
A seminal study of the ERG was conducted by Ragnar Granit (1955). He recognized the distributed nature of the sources and designed experiments to block different parts in an effort to identify the major elements contributing to the waveform. He deduced the presence of three main components, namely PI, PII, and PIII. PI is a slowly developing positive potential and is associated with the c-wave. PII is also positive but develops more rapidly and is chiefly responsible for the b-wave. PIII is the negative component; its initial phase develops rapidly and is associated with the onset of the a-wave. The total ERG is found by superposition (summing) of PI+PII+PIII.
 A seminal study of recordings from different retinal layers and individual retinal cells was later made also by Torsten Wiesel (Swedish, 1924-) and K.T. Brown (1961). Torsten Nils Wiesel shared the 1981 Nobel Prize with David Hunter Hubel "for discoveries concerning information processing in the visual system."
A seminal study of recordings from different retinal layers and individual retinal cells was later made also by Torsten Wiesel (Swedish, 1924-) and K.T. Brown (1961). Torsten Nils Wiesel shared the 1981 Nobel Prize with David Hunter Hubel "for discoveries concerning information processing in the visual system."
 The scientific works of Ragnar Granit are well summarized in Granit (1955). It includes also a large list of references to his works in vision and in other fields of bioelectromagnetism and neurophysiology..
The scientific works of Ragnar Granit are well summarized in Granit (1955). It includes also a large list of references to his works in vision and in other fields of bioelectromagnetism and neurophysiology..
REFERENCES
du Bois-Reymond EH (1848): Untersuchungen Ueber Thierische Elektricität, Vol. 1, 56+743 pp. G Reimer, Berlin.
Carpenter RHS (1988): Movements of the Eyes, 2nd ed., 593 pp. Pion, London.
Charles S (1979): Electrical signals of the retinal microcircuitry. In Physiology of the Human Eye and Visual System, ed. RE Records, pp. 319-27, Harper & Row, Hagerstown.
Clark JW (1978): The electroretinogram. In Medical Instrumentation, ed. JG Webster, pp. 177-84, Houghton Mifflin, Boston.
Dewar J, McKendrick JG (1873): On the physiological action of light. Proc. Roy. Soc. (Edinburgh) 8: 179-82.
Doslak MJ (1988): Electroretinography. In Encyclopedia of Medical Devices and Instrumentation, Vol. 2, ed. JG Webster, pp. 1168-80, John Wiley, New York.
Doslak MJ, Hsu P-C (1984): Application of a bioelectric field model of the ERG to the effect of vitreous haemorrhage. Med. & Biol. Eng. & Comput. 22: 552-7.
Doslak MJ, Plonsey R, Thomas CW (1980): The effects of variations of the conducting media inhomogeneities on the electroretinogram. IEEE Trans. Biomed. Eng. 27: 88-94.
Doslak MJ, Plonsey R, Thomas CW (1982): Numerical solution of the bioelectric field. Med. & Biol. Eng. & Comput. 19: 149-56.
Granit R (1955): Receptors and Sensory Perception, 369 pp. Yale University Press, New Haven.
Holmgren F (1865): Method att objectivera effecten af ljusintryck på retina. Uppsala Läk. För. Förh. 1: 184-98.
Levick WR, Dvorak DR (1986): The retina - From molecules to networks. Trends Neurosci. 9: 181-5.
Oster PJ, Stern JA (1980): Electro-oculography. In Techniques in Psychophysiology, ed. I Martin, PH Venables, pp. 276-97, John Wiley, New York.
Rahko T, Karma P, Torikka T, Malmivuo JA (1980): Microprocessor-based four-channel electronystagmography system. Med. & Biol. Eng. & Comput. 18:(1) 104-8.
Rodieck RW (1973): The Vertebrate Retina, 1044 pp. Freeman, San Francisco.
Stockwell CW (1988): Nystagmography. In Encyclopedia of Medical Devices and Instrumentation, Vol. 3, ed. JG Webster, pp. 2090-4, John Wiley, New York.
Wiesel TN, Brown KT (1961): Localization of origins of electroretinogram components by intraretinal recording in the intact cat eye. J. Physiol. (Lond.) 158: 257-80.
Young LR, Sheena D (1975): Eye movement measurement techniques. Amer. Physiologist 30: 315-30. (Reprinted in: Encyclopedia of Medical Devices and Instrumentation, Webster, JG, ed., J. Wiley & Sons, New York, vol. 2., pp. 1259-1269, 1988).
Young LR, Sheena D (1988): Eye-movement measurement techniques. In Encyclopedia of Medical Devices and Instrumentation, ed. JG Webster, pp. 1259-69, John Wiley, New York.
FURTHER READING
Berthoz A, Melvill Jones G (1985): Adaptive mechanisms in gaze control. In Reviews of Oculomotor Research, Vol. 1, ed. DA Robinson, H Collewjin, p. 386, Elsevier, Amsterdam.
Büttner-Ennever JA (1989): Neuroanatomy of the oculomotor system. In Reviews of Oculomotor Research, Vol. 2, ed. DA Robinson, H Collewjin, p. 478, Elsevier, Amsterdam.
Kowler E (1990): Eye movements and their role in visual and cognitive processes. In Reviews of Oculomotor Research, Vol. 4, ed. DA Robinson, H Collewjin, p. 496, Elsevier, Amsterdam.
Wurtz RH, Goldberg ME (1989): The neurobiology of saccadic eye movements. In Reviews of Oculomotor Research, Vol. 3, ed. DA Robinson, H Collewjin, Elsevier, Amsterdam.


